大会抢先看!2025 AASLD:乙肝领域有什么最新动态?
时间:2025-11-04 12:09:01 热度:37.1℃ 作者:网络
2025年11月7日-11日,全球肝病领域顶级学术盛会-美国肝病研究学会(AASLD)年度大会 The Liver Meeting 2025 将在美国•华盛顿会议中心正式召开。本次会议将汇聚肝病研究领域的最新科研硕果与前沿探索方向,为全球参会者精心构筑一个深化肝病理论认知、拓展专业视野边界的学术交流殿堂。
为助力广大读者精准捕捉会议的学术精髓,及时洞悉领域前沿动态,肝胆相照平台特别甄选会议摘要中的热点研究内容,本篇专题报道将聚焦“乙型肝炎”这一关键领域,为广大同仁提供最新研究进展,展望其在临床实践中的广阔应用前景。
一 慢性乙型肝炎患者首次接受恩替卡韦(ETV)与丙酚替诺福韦(TAF)治疗后肝细胞癌(HCC)长期发病率相似:REAL-B研究
Similar Long-Term Incidence of Hepatocellular Carcinoma (HCC) in Chronic Hepatitis B Patients Who Initiated Entecavir (ETV) Versus Tenofovir Alafenamide (TAF): A REAL-B Study
作者:Haruki Uojima¹,Rui Huang¹,Mindie Nguyen¹,Huy Trinh²,Akito Nozaki³,Philip Vutien⁴,Hidenori Toyoda⁵,Takashi Honda⁶,Hiroshi Abe⁷,Masanori Atsukawa⁸,Tsunamasa Watanabe⁹,Toru Ishikawa¹⁰,Masaru Enomoto¹¹,Yasuhito Tanaka¹²,Koichi Takaguchi¹³,Pei-Chien Tsai⁴,Mayumi Maeda¹,Ramsey Cheung¹,Takanori Ito⁶,Manabu Morimoto³,Ming-Lung Yu¹⁴,Chung-Feng Huang⁴,Wan Long Chuang⁴,Chia-Yen Dai⁴,Ming-Lun Yeh⁴,Chao Wu¹⁵,Tomonori Senoh¹³,Jee-Fu Huang⁴
¹斯坦福大学医学中心;²圣何塞胃肠病中心;³横滨市立大学医疗中心;⁴高雄医科大学;⁵大垣市立医院;⁶名古屋大学医学院;⁷新松户综合医院;⁸日本医科大学;⁹圣玛利安娜医科大学;¹⁰济生会新潟医院;¹¹大阪公立大学;¹²熊本大学;¹³香川县立中央医院;¹⁴高雄医科大学附属医院;¹⁵南京大学
背景
慢性乙型肝炎(CHB)仍是全球重要的公共卫生问题,长期抗病毒治疗可显著降低肝细胞癌(HCC)的发生风险。恩替卡韦(ETV)与丙酚替诺福韦(TAF)均为推荐的一线抗病毒药物,具有强效的病毒抑制能力和良好的安全性。
既往关于ETV与富马酸替诺福韦二吡呋酯(TDF)在HCC预防方面的研究结果存在分歧,但在充分校正治疗时期与患者基线特征差异后,多数研究提示两者差异并不显著。然而,ETV与TAF在相同治疗时期内的长期比较数据仍较为有限。本研究旨在评估2016年TAF获批后,同一治疗时期内ETV与TAF初始治疗CHB患者的长期HCC风险。
Background:Chronic hepatitis B (CHB) remains a major global health burden, and long-term antiviral therapy can reduce the risk of hepatocellular carcinoma (HCC). Entecavir (ETV) and tenofovir alafenamide (TAF) are both first-line therapies with potent viral suppression and favorable safety profiles. Previous comparisons between ETV and tenofovir disoproxil fumarate (TDF) have yielded conflicting results regarding HCC prevention. However, data adequately adjusted for differences in treatment eras and patient characteristics generally show no significant difference. Comparative data for ETV versus TAF remain limited.
We aimed to investigate the long-term HCC risk in ETV versus TAF users during the same treatment era following TAF approval in 2016.
方法
本研究纳入REAL-B联盟来自7个国家24个中心的既往未接受抗病毒治疗的CHB患者,这些患者于2016年至2023年间开始接受ETV或TAF治疗。排除合并其他病毒感染或既往恶性肿瘤者。主要结局为HCC发生率,采用Fine和Gray竞争风险模型进行分析。
为平衡患者基线特征,采用治疗倾向逆概率加权(IPTW)方法,并进行倾向评分匹配(PSM)敏感性分析。匹配变量包括年龄、性别、种族、饮酒情况、肝硬化、肝脂肪变性、HBsAg水平、HBV DNA、血小板计数、AST、ALT及白蛋白。
Methods:We enrolled previously treatment-naïve CHB patients from 24 centers across 7 countries in the REAL-B consortium who initiated either ETV or TAF between 2016 and 2023. Patients with viral co-infections or prior malignancy were excluded.The primary outcome was HCC incidence, analyzed using Fine and Gray competing risk models. To balance patient characteristics, we applied inverse probability of treatment weighting (IPTW), and conducted sensitivity analyses using propensity score matching (PSM). Matching variables included age, sex, race, alcohol use, cirrhosis, hepatic steatosis, HBsAg, HBV DNA, platelet count, AST, ALT, and albumin.
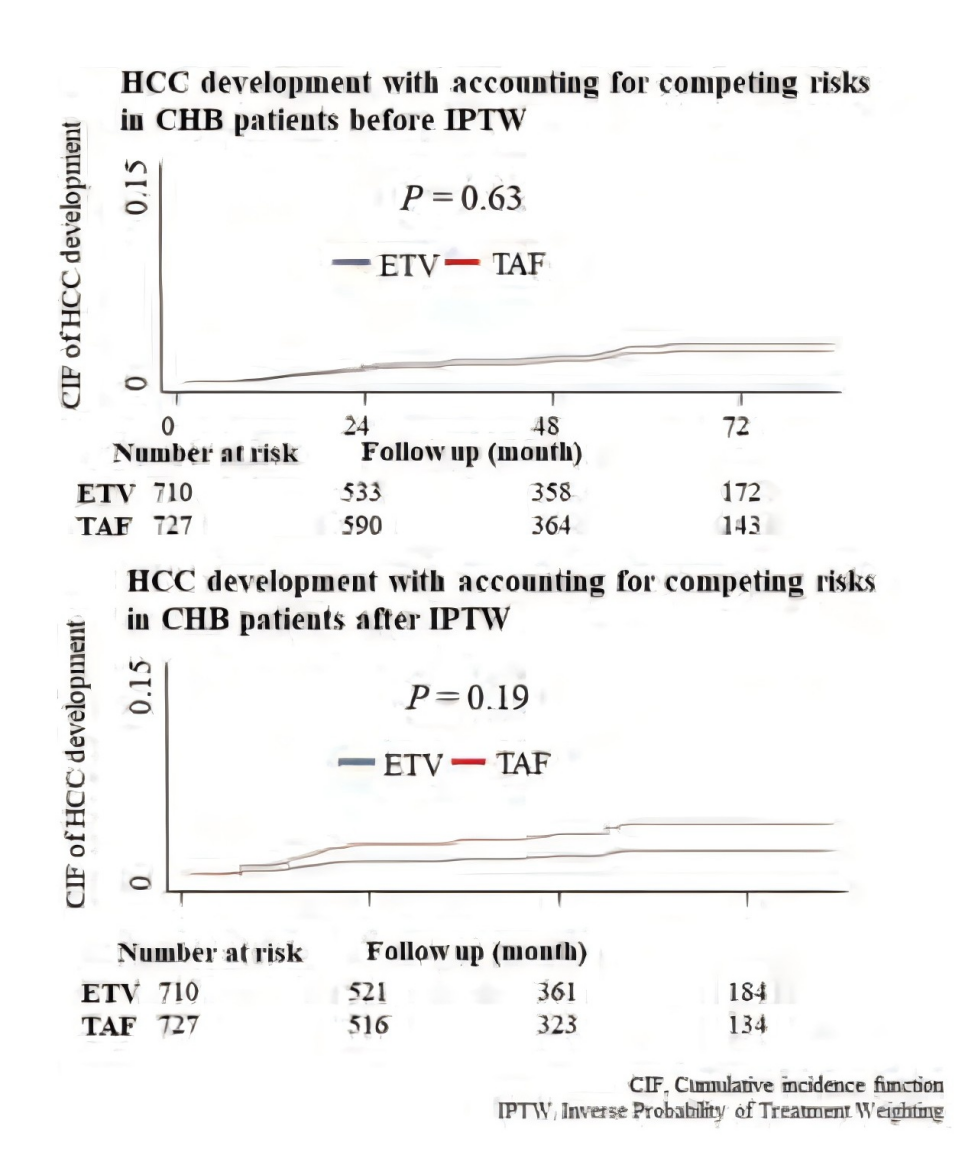
结果
共纳入1,437例患者,其中ETV组710例,TAF组727例。两组平均随访时间相似(48.5±25.0个月 vs 48.7±22.5个月,P=0.84)。匹配前,ETV组患者年龄较大,男性及肝硬化比例更高。IPTW后,两组基线特征平衡(所有标准化差异均 < 0.1)。
随访期间,ETV组12例、TAF组14例患者发生HCC,发病率分别为0.10例/100人年和0.18例/100人年(P=0.19)。ETV与TAF组HCC风险无显著差异[亚分布风险比 (SHR):2.17;95% CI:0.68–6.85;P=0.19]。按年龄、性别、肝硬化、肝脂肪变性、HBeAg、ALT及HBV DNA水平分层的亚组分析,以及PSM敏感性分析结果一致。
Results:A total of 1,437 patients were included (710 ETV, 727 TAF) with similar mean follow-up durations (48.5 ± 25.0 vs. 48.7 ± 22.5 months, P = 0.84). Before matching, ETV patients were significantly older and more likely to be male or have cirrhosis. After IPTW, baseline characteristics were balanced between groups (all standardized differences < 0.1). During follow-up, HCC occurred in 12 ETV patients and 14 TAF patients, corresponding to incidence rates of 0.10 and 0.18 per 100 person-years, respectively (P = 0.19). There was no statistically significant difference in HCC risk between ETV and TAF (subdistribution hazard ratio [SHR]: 2.17; 95% CI: 0.68–6.85; P = 0.19). Consistent results were observed across subgroups stratified by age, sex, cirrhosis, hepatic steatosis, HBeAg, ALT, and HBV DNA levels, and in sensitivity analyses using PSM.
结论
在这项多国多中心的真实世界研究中,2016年后同期开始ETV或TAF治疗的既往未治疗CHB患者,其HCC长期发病率无显著差异。多种统计分析方法与亚组结果均一致,提示ETV与TAF在HCC预防方面疗效相当。因此,临床治疗方案的选择应主要基于药物成本及患者个体化因素进行综合考量。
Conclusion:In this real-world, multinational study of previously untreated CHB patients who initiated antiviral therapy during the same treatment era, HCC incidence did not differ significantly between ETV and TAF groups.Findings were consistent across multiple analytic approaches and patient subgroups, suggesting that both agents offer comparable protection against HCC. Therapeutic selection should therefore be based on cost considerations and individual patient factors.
二 低基线HBsAg滴度HBeAg阴性、非肝硬化慢性乙型肝炎患者停药后的结局:ADAPT试验
Outcomes Following Antiviral Therapy Discontinuation in HBeAg-Negative, Non-Cirrhotic Chronic Hepatitis B Patients with Low Baseline HBsAg Titers: The ADAPT Trial
作者:Jonggi Choi¹、Won-Mook Choi²、Neung Hwa Park³、Gi-Ae Kim⁴、Ji Hoon Kim⁵、Young-Suk Lim²
¹韩国首尔蔚山大学峨山医疗中心;²韩国首尔蔚山大学医学院峨山医疗中心;³韩国蔚山大学医学院蔚山医院;⁴韩国首尔庆熙大学医院;⁵韩国首尔高丽大学九老医院
背景
对于慢性乙型肝炎(CHB)患者而言,停止抗病毒治疗的获益与风险(包括持续病毒学应答及HBsAg血清学清除)仍存在争议。
Background:The benefits and risks of antiviral therapy discontinuation, including sustained virologic response and HBsAg seroclearance, remain controversial in chronic hepatitis B (CHB) patients.
方法
本研究为多中心、单臂停药试验(ADAPT试验,NCT04782375),纳入来自韩国四家医疗中心的HBeAg阴性、非肝硬化CHB患者,这些患者于2021年9月至2023年6月期间停止抗病毒治疗[富马酸替诺福韦二吡呋酯(TDF)、替诺福韦艾拉酚胺(TAF)、恩替卡韦(ETV)或其他]。
纳入标准包括:抗病毒治疗≥2年、停药时HBV DNA不可检测(<20IU/mL)、血清HBsAg滴度<3,000IU/mL及丙氨酸氨基转移酶(ALT)<40U/L。停药后随访每4周进行一次至第24周,之后间隔延长至第48周。主要终点为第24周病毒反弹(HBV DNA ≥2,000 IU/mL)发生率;次要终点包括第48周的HBsAg清除率及再治疗率。
Methods:This multicenter, single-arm discontinuation trial (ADAPT trial, NCT04782375) enrolled HBeAg-negative, non-cirrhotic CHB patients from four centers in Korea who discontinued antiviral therapy (tenofovir disoproxil fumarate [TDF], tenofovir alafenamide [TAF], entecavir [ETV], or others) between September 2021 and June 2023. Eligible patients had received antiviral treatment for at least 2 years, had undetectable HBV DNA (<20 IU/mL), serum HBsAg titers <3,000 IU/mL, and alanine aminotransferase (ALT) levels <40 U/L at the time of discontinuation. Follow-up visits occurred every 4 weeks until week 24, and less frequently until week 48. The primary endpoint was viral rebound (HBV DNA ≥2,000 IU/mL) at week 24. Secondary endpoints included HBsAg seroclearance and retreatment rates at week 48.
结果
共纳入140例患者(平均年龄52.6岁,男性占73.6%),停药时所用药物包括TDF(58.6%)、TAF(7.1%)、ETV(29.3%)及其他(5.0%)。基线时中位HBsAg滴度为439IU/mL(IQR100–839),平均ALT为22U/L。共有9例患者中途退出,131例完成24周随访。第24周时,84例(60.0%)患者出现病毒反弹,其中83例重新启动抗病毒治疗;在未发生病毒反弹的56例患者中,14例(25.0%)因预设标准重新接受治疗。
总体而言,第24周时共有97例(69.3%)患者恢复抗病毒治疗,34例(24.3%)维持停药状态。至第48周,7例(5.0%)患者出现HBsAg血清学清除,且均为基线HBsAg滴度<300IU/mL的患者;此时共有104例(74.3%)患者恢复治疗,27例(19.3%)仍维持停药状态。另有3例患者出现ALT急剧升高(≥正常上限5倍),但均未出现肝功能失代偿。
Results:Of 140 patients (mean age 52.6 years, 73.6% male), the antiviral treatment at discontinuation included TDF (58.6%), TAF (7.1%), ETV (29.3%), and others (5.0%). Median baseline HBsAg titer was 439 IU/mL (IQR 100–839), and mean ALT was 22 U/L. Nine patients withdrew, and 131 completed 24-week follow-up. At week 24, viral rebound occurred in 84 (60.0%) patients, of whom 83 resumed antiviral therapy. Among 56 patients without viral rebound, 14 (25.0%) resumed therapy based on pre-defined criteria. Consequently, by week 24, 97 patients (69.3%) resumed antiviral therapy, while 34 patients (24.3%) remained off-treatment. By week 48, HBsAg seroclearance occurred in 7 patients (5.0%), all with baseline HBsAg titers <300 IU/mL. At week 48, 104 patients (74.3%) resumed antiviral therapy, and 27 patients (19.3%) remained off-treatment. Three patients experienced ALT flares (ALT ≥5× the upper limit of normal) without hepatic decompensation.
结论
在这项纳入140例非肝硬化、HBeAg阴性、基线HBsAg滴度<3,000IU/mL的多中心试验中,约74.3%的患者在停药48周内因病毒反弹需重新治疗,仅5.0%的患者实现HBsAg清除,且均为基线HBsAg滴度<300IU/mL者。结果提示,目前不支持对大多数慢性乙型肝炎患者普遍实施抗病毒治疗停药策略。
Conclusion:In this multicenter trial of 140 non-cirrhotic, HBeAg-negative CHB patients with baseline HBsAg titers <3,000 IU/mL, 74.3% required retreatment by week 48 due primarily to viral rebound. Only 5.0% achieved HBsAg seroclearance, exclusively among patients with baseline HBsAg titers <300 IU/mL. These findings do not support universal discontinuation of antiviral therapy for most CHB patients.
三 从既往核苷(酸)类似物转换为替诺福韦艾拉酚胺(TAF)后对慢性乙型肝炎患者血脂谱及心血管风险的影响
EFFECT OF SWITCHING FROM PRIOR NUCLEOS(T)IDE ANALOGUE(S) TO TENOFOVIR ALAFENAMIDE ON LIPID PROFILE AND CARDIOVASCULAR RISK IN PATIENTS WITH CHRONIC HEPATITIS B
作者:Witchayaporn Praguylertluck¹,Pimsiri Sripongpun¹,Apichat Kaewdech¹,Naichaya Chamroonkul¹
¹Prince of Songkla University
背景
替诺福韦艾拉酚胺(Tenofovir alafenamide, TAF)被推荐作为慢性乙型肝炎(CHB)的一线治疗药物。然而,既往研究发现,从替诺福韦二吡呋酯(Tenofovir disoproxil fumarate, TDF)转换至TAF的患者中,低密度脂蛋白胆固醇(LDL-c)水平可能升高。而关于从其他抗病毒药物转换至TAF后的血脂及心血管结局数据仍较为有限。本研究旨在探讨CHB患者转换至TAF后对血脂及心血管风险的影响。
Background:Tenofovir alafenamide (TAF) is recommended as the first-line treatment for chronic hepatitis B (CHB). However, increased LDL-c levels have been observed in patients switched from Tenofovir disoproxil fumarate (TDF) to TAF, while data on switching from other antiviral agents to TAF remain limited. We investigated how switching to TAF affected lipid and cardiovascular outcomes in CHB patients.
方法
本研究为前瞻性观察性研究,纳入根据泰国国家医保政策于2022年底由既往核苷(酸)类似物(NUC)转换至TAF治疗的CHB患者。所有入组患者在转换时(基线,0周)及转换48周后接受血脂检测和瞬时弹性成像(Transient Elastography, TE)检查。收集患者人口学资料、既往NUC用药、肝生化指标、受控衰减参数(CAP)及肝硬度(E值)数据。比较基线与48周后血脂、泰国心血管(CV)风险评分及TE指标的变化。
Methods:We conducted a prospective observational study including CHB patients who had to switch from their prior nucleos(t)ide analogue (NUC) to TAF according to the Thai national reimbursement policy in late 2022. All enrolled patients had lipid tests and transient elastography (TE) at baseline (0 week) and 48 weeks post-switch to TAF. Demographic data, prior NUC, liver biochemistry, controlled attenuation parameter (CAP), and liver stiffness (elastic modulus; E) data measured by TE were collected. Changes in lipid profile, Thai cardiovascular (CV) risk score, and TE results between baseline and 48 weeks were compared.
结果
共有110例转换至TAF并完成48周随访的患者纳入分析。既往NUC用药如下:拉米夫定(Lamivudine, LAM)47例,恩替卡韦(Entecavir, ETV)2例,TDF方案41例。三组患者的基线特征总体相似,但TDF组中高血压比例较高且基线总胆固醇水平较低。
在转换至TAF 48周后,三组患者的中位LDL-c变化分别为:ETV组−2.45 mg/dL,LAM组−5.9 mg/dL,TDF组+8.8 mg/dL(p<0.001);总胆固醇变化分别为−4.5、−4和+17 mg/dL(p<0.001);体重变化分别为0、0和+1 kg(p=0.010)。
肝脏脂肪变(CAP测量)、肝硬度(E值)及泰国心血管风险评分的变化在三组之间无显著差异。随访期间未发生心血管事件。
多因素逻辑回归分析结果显示:在转换至TAF前使用TDF方案是LDL-c恶化(定义为LDL-c水平升高或降脂药剂量/强度增加)的独立危险因素,调整体重指数(BMI)变化、年龄、性别及基线高血压后,其校正比值比为3.84(95% CI 1.55–10.22,p=0.005)。
Results:A total of 110 patients who were switched to TAF and completed 48-week follow-up were analyzed. The prior NUCs were as follows: 47 Lamivudine (LAM), 2 Entecavir (ETV), and 41 TDF-based regimens. Baseline characteristics were similar among the three groups, except that hypertension was more frequent and baseline total cholesterol levels were lower in the TDF-based group.
At 48 weeks post-switch, the median LDL-c changes were −2.45, −5.9, and +8.8 mg/dL (p<0.001); total cholesterol changes were −4.5, −4, and +17 mg/dL (p<0.001); and weight changes were 0, 0, and +1 kg (p=0.010) in the ETV, LAM, and TDF-based groups, respectively. Changes in hepatic steatosis (measured by CAP), liver stiffness (E), and Thai CV risk score were not significantly different. No cardiovascular events occurred during follow-up.
Multivariable logistic regression analysis confirmed that a TDF-based regimen at baseline, prior to the switch to TAF, was significantly associated with worsening LDL-c (defined as an increase in LDL-c level or increased dose/intensity of lipid-lowering agent), with an adjusted odds ratio of 3.84 (95% CI 1.55–10.22, p=0.005), after adjustment for BMI change, age, sex, and baseline hypertension.
结论
仅在既往接受TDF治疗的患者中,转换至TAF后观察到LDL-c和总胆固醇显著升高,而在既往使用ETV或LAM的患者中未见类似变化。因此,转换至TAF后的血脂监测可能无需普遍实施。仍需进一步研究TAF长期使用后的心血管结局。
Conclusion: A significant increase in LDL-c and total cholesterol after switching to TAF was observed only in patients previously treated with TDF, but not in those with prior ETV or LAM. Therefore, careful lipid monitoring after switching to TAF may not be universally required. Long-term cardiovascular outcome data are warranted.


