【论著】| 荧光原位杂交与尿脱落细胞学在尿路上皮癌诊断中的效能比较:一项单中心回顾性队列研究
时间:2025-01-24 12:09:16 热度:37.1℃ 作者:网络
[摘要] 背景与目的:尿路上皮癌是泌尿系统常见的恶性肿瘤,其早期诊断对改善患者预后至关重要。本研究比较了荧光原位杂交(fluorescence in situ hybridization,FISH)、尿脱落细胞学及二者联合检测在尿路上皮癌及其不同亚型中的诊断效能。方法:本研究纳入了2022年1月—2023年12月行经尿道膀胱肿瘤切除术(transurethral rep of bladder tumor, TURBT)且符合入组标准及排除标准的患者,且获得复旦大学附属肿瘤医院伦理委员会批准(伦理批号:050432-4-2307E)。收集患者本次TURBT后病理学诊断结果及术前1周的FISH和脱落细胞学检测结果,对FISH、脱落细胞学及二者联合检测在尿路上皮癌中的诊断准确率、灵敏度和特异度进行统计学分析。本研究属于观察性研究,严格遵循《加强流行病学中观察性研究报告质量》(Strengthening the Reporting of Observational Studies in Epidemiology,STROBE)指南及《诊断准确性研究报告规范》(Standards for Reporting of Diagnostic Accuracy,STARD)中的各项条目。结果:本研究共纳入283例TURBT术后患者,其中136例为尿路上皮癌,147例为良性病变。136例尿路上皮癌中,根据病理学亚型分组, 79(58.09%)例为浸润性尿路上皮癌,57(41.91%)例为非浸润性尿路上皮癌。根据恶性程度分组,112(82.35%)例为高级别尿路上皮癌,24(17.65%)例为低级别尿路上皮癌。以组织病理学诊断结果为金标准,136例尿路上皮癌中FISH、脱落细胞学、联合检测的准确率分别为79.51%、72.08%、77.39%,灵敏度分别为72.06%、58.82%、78.68%,特异度分别为86.39%、84.35%、76.19%。FISH、联合检测的曲线下面积(area under the curve,AUC)差异无统计学意义,但均高于脱落细胞学(0.792 vs 0.716,P=0.006;0.774 vs 0.716,P=0.004);FISH与脱落细胞学相比,净重分类改善(net reclassification improvement,NRI)提升15.28%(P=0.006)。79例浸润性尿路上皮癌中,FISH的准确率高于脱落细胞学(86.28% vs 78.32%,P=0.011);FISH、联合检测的灵敏度均高于脱落细胞学(86.08% vs 67.09%,P=0.004;91.14% vs 67.09%,P<0.001),AUC值也高于脱落细胞学(0.808 vs 0.713,P=0.004;0.784 vs 0.713,P=0.007);FISH与脱落细胞学相比,NRI值提升21.03%(P=0.003)。57例非浸润性尿路上皮癌中,3种检测方法的AUC值均较低(AUC<0.700)。112例高级别尿路上皮癌中,FISH的准确率、联合检测的灵敏度均高于脱落细胞学(84.94% vs 76.45%,P=0.005;89.29% vs 66.07%,P<0.001),FISH及联合检测的AUC值也均高于脱落细胞学(0.847 vs 0.752,P=0.002;0.827 vs 0.752,P=0.001),FISH比脱落细胞学的NRI值提升19.01%(P=0.003)。24例低级别尿路上皮癌中,3种检测方法的AUC值均较低(AUC<0.600)。结论:尿路上皮癌中,尤其是浸润性和高级别尿路上皮癌,FISH的诊断效能优于脱落细胞学;FISH单独检测的准确率、灵敏度与联合检测相当,且特异性高于联合检测。在非浸润性或低级别尿路上皮癌中,3种检测方法的诊断效能均较低。
[关键词] 尿路上皮癌;荧光原位杂交;脱落细胞学;诊断效能;受试者工作曲线
[Abstract] Background and purpose: Urothelial carcinoma (UC) is a prevalent malignant tumor of the urinary system, and early diagnosis is crucial for improving patient prognosis. This study evaluated the diagnostic efficacy of fluorescence in situ hybridization (FISH), urine cytology and their combination for UC, as well as for its different subtypes. Methods: This study included patients who underwent transurethral rep of bladder tumor (TURBT) from January 2022 to December 2023 and approved by Ethics Commetce of Fudan Univesity Shanghai Cancer Center, No.: 050432-4-2307E) that met the inclusion and exclusion criteria. We collected TURBT pathological results and pre-procedure FISH and cytology results. Diagnostic accuracy, sensitivity and specificity of FISH, cytology and their combination were analyzed and compared for urothelial carcinoma. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist and Standards for Reporting of Diagnostic Accuracy (STARD) were followed for this study. Results: A total of 283 patients were enrolled in this study, 136 were diagnosed with UC, and 147 were not. Of the 136 UC cases, 79 (58.09%) were invasive and 57 (41.91%) were non-invasive. In terms of malignancy grade, 112 (82.35%) were high-grade UC and 24 (17.65%) were low-grade UC. Using histopathology as the gold standard, the accuracy of FISH, cytology and their combination in diagnosing UC was 79.51%, 72.08% and 77.39%, respectively; sensitivity was 72.06%, 58.82% and 78.68%, respectively; specificity was 86.39%, 84.35% and 76.19%, respectively. The area under the curve (AUC) for FISH and the combination was similar but higher than that for cytology (0.792 vs 0.716, P=0.006; 0.774 vs 0.716, P=0.004); the Net Reclassification Improvement (NRI) for FISH compared to cytology was 15.28% (P=0.006). In the 79 cases of invasive UC, FISH had higher accuracy than cytology (86.28% vs 78.32%, P=0.011). The sensitivity of FISH and the combination was higher than that of cytology (86.08% vs 67.09%, P=0.004; 91.14% vs 67.09%, P<0.001), and the AUC values were also higher (0.808 vs 0.713, P=0.004; 0.784 vs 0.713, P=0.007). The NRI for FISH compared to cytology was 21.03% (P=0.003). In the 57 cases of non-invasive UC, the AUC values for all three methods were low (AUC<0.700). Among the 112 cases of high-grade UC, FISH had higher accuracy (84.94% vs 76.45%, P=0.005), and the combination had higher sensitivity (89.29% vs 66.07%, P<0.001) compared to cytology. The AUC values for FISH and the combination were also superior to that for cytology (0.847 vs 0.752, P=0.002; 0.827 vs 0.752, P=0.001). The NRI for FISH compared to cytology was 19.01% (P=0.003). In the 24 cases of low-grade UC, the AUC values for all three methods were low (AUC<0.600). Conclusion: For UC, particularly invasive and high-grade subtypes, FISH shows superior diagnostic efficacy compared to cytology. FISH alone offers accuracy and sensitivity comparable to the combination test, with higher specificity. In cases of non-invasive or low-grade UC, however, all three diagnostic methods demonstrate relatively low efficacy.
[Key words] Urothelial carcinoma; Fluorescence in situ hybridization (FISH); Urine cytology; Diagnostic efficacy; Receiver operating characteristic curve
尿路上皮癌(urothelial carcinoma,UC)是一种起源于尿路内壁细胞的侵袭性恶性肿瘤,常见于膀胱、肾盂、输尿管和尿道[1]。尿路上皮癌具有起病隐匿、高复发和高死亡率的特点,因此,早期诊断对尿路上皮癌患者的预后至关重要[2]。目前,临床上主要采用膀胱镜检查、输尿管镜检查、超声和尿脱落细胞学检查(urine cytology)来诊断尿路上皮癌[3]。膀胱镜检查是诊断尿路上皮癌的“金标准”,但该检查对微小或隐匿的病灶存在漏诊风险,并且部分患者不能耐受膀胱镜检查[4]。尿脱落细胞学作为一种非侵入性且操作简便的检查方法,通过分析尿液中脱落细胞的形态来判定肿瘤的存在,然而,其结果可能受到血尿、尿路感染或膀胱灌注化疗等因素的干扰,对诊断低级别尿路上皮癌的灵敏度较低[5-7]。因此,寻找无创且更精准的检测手段对尿路上皮癌的诊断具有重要意义。
荧光原位杂交(fluorescence in situ hybridization,FISH)技术利用荧光标记的特异性DNA探针与细胞有丝分裂中期或间期的靶DNA序列结合,形成带有荧光标记的异源双链DNA,能显著提升检测的敏感性与特异性[8]。尿路上皮癌常发生非随机染色体改变,包括染色体的结构异常和数目异常[9]。Sokolova等[10]研究发现,FISH能通过特异性识别3、7、17号染色体和9p21位点的异常来诊断尿路上皮癌,同时具有无创、快速和高效等优点。研究[11-13]表明, FISH在诊断尿路上皮癌方面的性能优于尿脱落细胞学,然而,FISH和尿脱落细胞学在不同浸润程度或病理学类型的尿路上皮癌中的检测性能差异仍需进一步研究。本研究旨在比较FISH、尿脱落细胞学及其联合应用在尿路上皮癌诊断中的效能,并评估这些检测方法在尿路上皮癌中的临床应用价值。
1 资料和方法
1.1 一般资料
本研究获得复旦大学附属肿瘤医院医学伦理委员会的批准(伦理批号:050432-4-2307E)。本研究收集了2022年1月—2023年12月在复旦大学附属肿瘤医院因尿路症状(血尿、排尿困难、膀胱刺激征)就诊并接受经尿道膀胱肿瘤切除术(transurethral rep of bladder tumors,TURBT)的患者。研究的纳入标准为:术前1周内同时进行了尿脱落细胞学和Urovysion FISH检测。排除标准为:① 同时患有其他肿瘤。② TURBT术未完全切除尿路上皮肿瘤。③ TURBT术后尿路上皮癌复发。本研究最终纳入了283例接受TURBT的患者。
1.2 方法
1.2.1 组织病理学检查
经TURBT获取的组织标本经10%福尔马林固定液固定、二甲苯透明处理、梯度乙醇脱水、石蜡浸润后制成蜡块。切片厚度为3~4 μm,切片经过苏木精-伊红(H-E)染色后,由病理科医师进行诊断。
1.2.2 尿液玻片制备
收集50 mL尿液标本,以1 500~1 800 r/min离心10 min后保留底部沉淀。向沉淀中加入10 mL甲醇-冰醋酸溶液(比例为3∶1),在-20 ℃固定至少30 min。固定好的样本再次离心,保留沉淀并用0.1~2.0 mL固定液重悬,用滴片法制成尿液细胞玻片。玻片置于72 ℃下完全干燥至少60 min。
1.2.3 FISH检测
使用Urovysion FISH检测试剂盒(美国Abbott公司)进行检测。尿液细胞玻片在72 ℃下预处理10 min,用胃蛋白酶消化10 min,使用磷酸缓冲盐溶液(phosphate-buffered saline,PBS)、4%甲醛溶液、PBS依次浸泡处理(每次5 min)后,完全干燥玻片。滴加Urovysion探针,在原位杂交仪(美国ThermoFisher公司)中进行变性杂交(条件:80 ℃变性8 min,39 ℃ 杂交24~30 h)。杂交结束后,对玻片进行洗涤、干燥处理,使用4’6二脒基-2-苯基吲哚(4’,6-diamidino-2-phenylindole,DAPI)对玻片进行复染,由病理科医师在荧光显微镜下进行FISH判读。
1.2.4 脱落细胞学检测
采集男性尿液的中段尿,女性尿液的导管尿或中段尿。收集后2 h内完成尿液制片,不能及时制片可用95%乙醇保存。尿液离心沉淀后,取沉淀物涂片,干燥后进行巴氏染色,由病理科医师进行诊断。
1.2.5 结果判读
⑴ 组织病理学检查结果判读:依据世界卫生组织(World Health Organization,WHO)2004年版指南进行病理学分级。所有切片均由经验丰富的病理科医师进行评估和诊断。本研究阳性结果分组依据:本次组织病理学诊断结果明确描述为“尿路上皮癌”。阴性结果分组依据:本次组织病理学诊断结果明确描述中不包含“尿路上皮癌”及“低度恶性潜能的乳头状尿路上皮肿瘤”。
⑵ FISH结果判读:使用荧光显微镜(日本Olympus公司)进行判读。阳性判定标准如下:在25个细胞中,≥4个细胞内观察到3、7、17号染色体至少两种多倍体增加,或≥12个细胞内观察到9p21位点两个拷贝数均缺失。本研究阳性结果分组依据:FISH检测病理学诊断报告结论为 “Urovysion检测结果:阳性”。阴性结果分组依据:FISH检测病理学报告结论为“Urovysion检测结果:阴性”。
⑶ 尿脱落细胞学检测结果判读:使用倒置显微镜(德国Leica公司)进行判读。阳性判定标准为:细胞形态异常,见核质比不同程度增高、染色质粗糙且核轮廓不规则的异型细胞。本研究阳性结果分组依据:细胞病理学检查报告描述为“癌细胞”、“疑癌细胞”、“非典型尿路上皮细胞”。阴性结果分组依据:细胞病理学诊断报告中描述为“未见肿瘤细胞”。
1.3 统计学处理
本研究分别计算FISH、尿脱落细胞学及FISH联合尿脱落细胞学法诊断尿路上皮癌的准确率、灵敏度、特异度、阳性预测值和阴性预测值。结果以百分比(%)表示,计算公式为:准确率=(真阳性+真阴性)/(真阳性+假阳性+真阴性+假阴性);灵敏度=真阳性/(真阳性+假阴性);特异度=真阴性/(真阴性+假阳性);阳性预测值=真阳性/(真阳性+假阳性);阴性预测值=真阴性/(真阴性+假阴性)。分类变量以频数或百分比(%)表示。使用McNemar配对χ2检验比较FISH、尿脱落细胞学、二者联合方法的诊断效能差异。对于多重比较,采用Bonferroni校正法(P=0.05/3)调整检验水准。采用受试者工作曲线(receiver operating characteristic curve,ROC)分析FISH、脱落细胞学法、FISH法联合脱落细胞学法的诊断效能,并通过曲线下面积(area under the curve,AUC)表示。3种方法的AUC值采用Z检验进行比较,统计量Z近似服从正态分布,计算公式为:
Z=
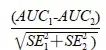
(其中SE1和SE2分别为AUC1和AUC2的标准误)。采用净重分类指数(Net Reclassification Improvement,NRI)评估FISH、联合检测法对整体诊断准确率的提升,对两种方法的NRI值采用Z检验进行比较,统计量Z近似服从正态分布,计算公式为:
Z=
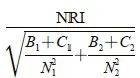
( 其中N1和N2分别表示患者组和良性病变组的总例数。B1表示患者组中,第一种方法检测正确而第二种方法检测错误的例数。C1表示患者组中,第一种方法检测错误而第二种方法检测正确的例数。B2表示良性病变中,第一种方法检测正确而第二种方法检测错误的例数。C2表示良性病变中,第一种方法检测错误而第二种方法检测正确的例数)。所有统计分析采用双侧检验,P<0.05为差异有统计学意义。数据处理和统计分析采用SPSS 26.0统计软件。
2 结 果
2.1 患者基本信息
本研究纳入了283例尿道膀胱肿瘤切除术后的患者,136例经病理学诊断为尿路上皮癌,147例为良性病变。本研究的流程图见图1。在136例尿路上皮癌中,有79例(58.09%)为浸润性尿路上皮癌,57例(41.91%)为非浸润性尿路上皮癌。根据恶性程度分组,136例尿路上皮癌中有112(82.35%)例为高级别尿路上皮癌组,24例(17.65%)为低级别尿路上皮癌组。
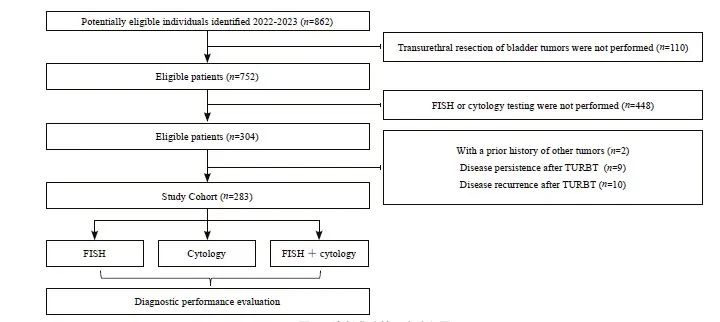
图1 诊断准确性研究流程图
Fig. 1 Flow diagram of a study on diagnostic accuracy
2.2 尿路上皮癌中3种检测方法的比较
本研究以组织病理学诊断结果为金标准,比较了尿路上皮癌中FISH、脱落细胞学及二者联合检测的诊断效能。FISH、脱落细胞学、联合检测的准确率分别为79.51%、72.08%、77.39%,灵敏度分别为72.06%、58.82%、78.68%,特异度分别为86.39%、84.35%、76.19%(表1)。
在38例FISH检测为假阴性的样本中,有11例为高级别浸润性尿路上皮癌,8例为高级别非浸润性尿路上皮癌,19例为低级别非浸润性尿路上皮癌。在56例脱落细胞学检测为阴性的尿路上皮癌样本中,26例为高级别浸润性尿路上皮癌,12例为高级别非浸润性尿路上皮癌,18例为低级别非浸润性尿路上皮癌。联合检测中有29例为假阴性,7例为高级别浸润性尿路上皮癌,5例为高级别非浸润性尿路上皮癌,17例为低级别非浸润性尿路上皮癌。
对20例FISH检测假阳性的样本进行分析,有12例活检组织的病理学诊断结果为尿路急性炎症期,1例含有坏死组织,1例前列腺增大,其余6例病理学诊断结果为慢性黏膜炎。23例经脱落细胞学检测为假阳性的样本中,5例活检组织的病理学诊断结果为尿路上皮异型增生,3例为尿路上皮异型增生伴黏膜坏死,2例为黏膜坏死伴钙化,1例为前列腺萎缩,其余12例病理学诊断结果为慢性黏膜炎。联合检测中有35例为假阳性,病理学诊断结果均为慢行黏膜炎。
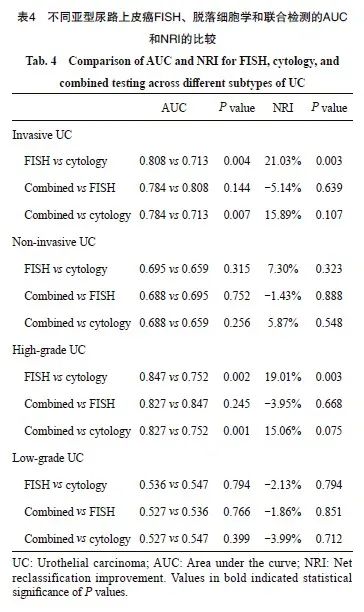
FISH、脱落细胞学、联合检测的AUC值分别为0.792、0.716和0.774(图2)。ROC曲线分析结果显示,FISH与联合检测差异无统计学意义(P=0.276),但FISH与脱落细胞学之间、联合检测与脱落细胞学之间的差异有统计学意义 (P=0.006,P=0.004,表2)。进一步对整体准确率提升进行进一步分析。结果显示,FISH比脱落细胞学的NRI值提升15.28%,两者之间差异有统计学意义(P=0.006,表2)。
在高级别尿路上皮癌的诊断中,仅有FISH与脱落细胞学之间的准确率差异有统计学意义(84.94% vs 76.45%,P=0.005)。灵敏度方面,联合检测高于脱落细胞学(89.29% vs 66.07%,P<0.001),但联合检测与FISH之间、FISH与脱落细胞学之间的灵敏度差异无统计学意义(89.29% vs 83.04%,P=0.023;83.04% vs 66.07%,P=0.700)。FISH、脱落细胞学、联合检测的AUC分别为0.847、0.752和0.827(图3、表3)。ROC曲线分析显示,FISH与脱落细胞学的AUC值差异、联合检测与脱落细胞学的AUC值差异均有统计学意义(P=0.002,P=0.001), FISH比脱落细胞学的NRI值提升了19.01%(P=0.003,表4)。在低级别尿路上皮癌中,3种检测方法的AUC值均小于0.600(图3D)。
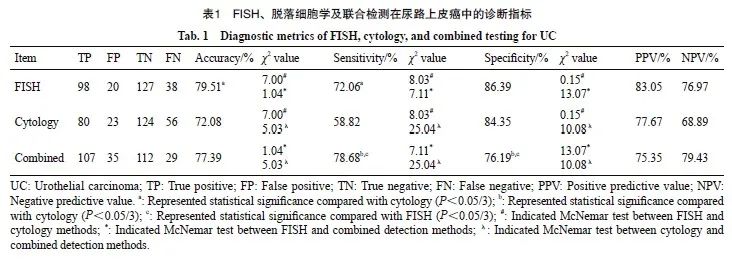
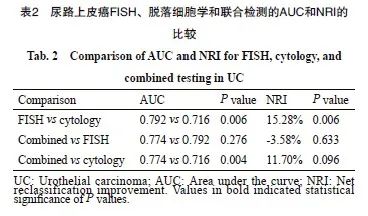
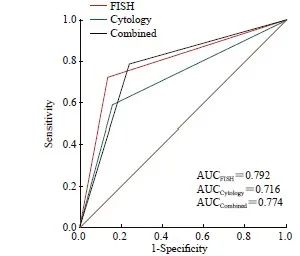
图2 尿路上皮癌中3种检测方法的诊断效能评价
Fig. 2 Diagnostic performance evaluation of the 3 methods in urothelial carcinoma
2.3 不同亚型尿路上皮癌中3种检测方法的比较
3种检测方法在不同亚型尿路上皮癌中的准确率、灵敏度、阳性预测值和阴性预测值见表3。在诊断浸润性尿路上皮癌时,FISH与脱落细胞学在准确率方面的差异有统计学意义(86.28% vs 78.32%,P=0.011)。在灵敏度方面,FISH与联合检测灵敏度差异无统计学意义,但均高于脱落细胞学(86.08% vs 67.09%,P=0.004;91.14% vs 67.09%,P<0.001)。FISH、脱落细胞学和联合检测的AUC分别为0.808、0.713和0.784(图3A)。FISH与脱落细胞学之间、联合检测与脱落细胞学之间的AUC值差异有统计学意义(P=0.004,P=0.007),FISH比脱落细胞学的NRI值差异提升了21.03%(P=0.003,表4)。在非浸润性尿路上皮癌中,3种检测方法的AUC值均小于0.700,并且3种检测方法之间的NRI值差异无统计学意义(图3B、表4)。
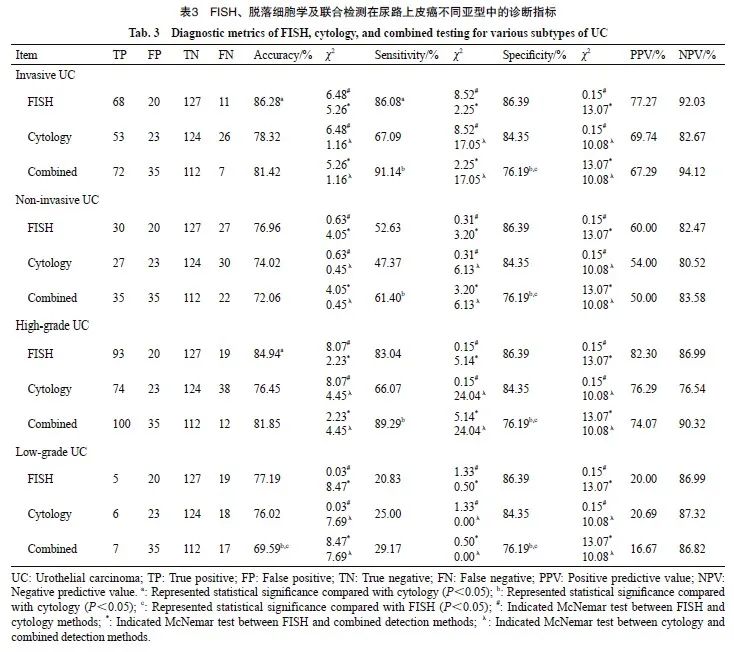
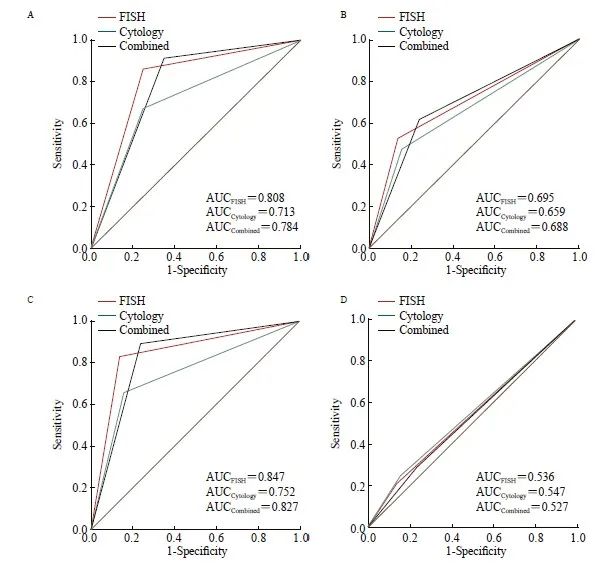
图3 不同亚型尿路上皮癌中3种检测方法的诊断性能评价
Fig. 3 Diagnostic performance evaluation of the three methods in the UC subtypes
A: Invasive UC; B: Non-invasive UC; C: High-grade UC; D: Low-grade UC. UC: Urothelial carcinoma.
3 讨 论
本研究比较了FISH、脱落细胞学及二者联合检测在尿路上皮癌诊断中的检测效能。结果显示,FISH的准确性和敏感性优于脱落细胞学,特异性相似,ROC曲线分析结果证实FISH的诊断效能优于脱落细胞学(0.792 vs 0.716,P=0.006)。这一研究与现有的研究结果一致, Aalami等[14]对13项研究进行了Meta分析,结果显示FISH的灵敏度为72%,特异度为95%。另一项Meta分析纳入了14项研究,共涉及2 031例患者。结果显示,FISH诊断尿路上皮癌的灵敏度明显高于脱落细胞学(84% vs 40%),而特异度相似(89.5% vs 95.9%)[15]。这些结果表明FISH在尿路上皮癌中的诊断效能优于脱落细胞学[16]。FISH联合脱落细胞学能提高尿路上皮癌检测的敏感性,但在实际应用中可能面临着假阳性率升高的问题[17-18]。本研究观察到联合检测的准确性和敏感性与FISH相似,敏感性高于脱落细胞学,但特异性低于FISH和脱落细胞学。联合检测特异性较低可能是由于尿液中存在染色体异常的非癌变细胞、尿液采集或处理不当导致细胞降解、细胞的异质性和肿瘤的复杂性、存在炎症或感染导致的细胞异常变化[19-20]。
根据病理学类型,尿路上皮癌可分为浸润性尿路上皮癌和非浸润性尿路上皮癌[21-22]。浸润性尿路上皮癌的恶性程度更高、更易发生转移,患者的5年生存率仅30%~40%[23]。研究[24]显示,FISH诊断浸润性尿路上皮癌的敏感性高于非浸润性尿路上皮癌(95% vs 65%)。本研究显示,与脱落细胞学相比,FISH在浸润性尿路上皮癌的诊断中具有更高的灵敏度(86.08% vs 67.09%,P=0.004)和更高的AUC值(0.808 vs 0713,P=0.004),且诊断的准确率提升了21.03%(P=0.003)。尽管联合检测的AUC值高于脱落细胞学,但其诊断准确率的提升不显著。这表明在浸润性尿路上皮癌的诊断中,即使不结合脱落细胞学,单独使用FISH也能提供较为可靠的结果。然而,在非浸润性尿路上皮癌的诊断中,3种检测方法均表现不佳,FISH、脱落细胞学和联合检测的AUC值均低于0.700。以往的研究[25-26]表明,单独使用FISH或脱落细胞学对非浸润性尿路上皮癌的诊断效果有限,这可能是因为非浸润性尿路上皮癌细胞通常局限于基底膜内,难以脱落,从而影响了检测的准确性。本研究进一步探讨了联合检测,结果显示,即使同时使用这两种方法,对非浸润性尿路上皮癌的诊断依然存在困难。
根据恶性程度,尿路上皮癌可分为低度恶性潜能的乳头状尿路上皮肿瘤、低级别的乳头状尿路上皮癌以及高级别的乳头状尿路上皮癌[27]。尿路上皮癌的恶性程度与肿瘤的侵袭性及复发风险密切相关[28]。脱落细胞学在高级别尿路上皮癌的诊断中表现出较高的特异性,但由于难以区分炎症反应与肿瘤复发,该方法的敏感性较 差[29]。FISH对高级别尿路上皮癌的阳性检出率明显高于低级别尿路上皮癌,其敏感性高于脱落细胞学,并且不受血尿、尿路感染或炎症的影响[30-31]。本研究中,FISH和联合检测在高级别尿路上皮癌中的灵敏度和AUC值较高(83.04%, AUC=0.847;89.29%,AUC=0.827),这与文献[32-33]报道的结果一致。进一步分析显示,尽管联合检测的AUC值高于脱落细胞学,但诊断准确率的提升并无统计学上的显著性意义。相比之下,FISH的诊断准确率显著提升,提示单独使用FISH在高级别尿路上皮癌的诊断中具有较高的临床价值。对于低级别尿路上皮癌,FISH、脱落细胞学和联合检测的AUC值均低于0.600,表明3种检测方法在诊断低级别尿路上皮癌时的效能均较差。FISH和脱落细胞学在这类癌症中较低的敏感性可能与低级别肿瘤细胞通常呈二倍体或近二倍体,且与正常细胞相似有关[34]。此外,低级别尿路上皮癌的假阴性结果可能是由于癌细胞缺乏、卡介苗(bacillus calmette guerin,BCG)治疗影响、炎症掩盖以及样本污染所致,而假阳性结果则可能主要归因于大量伞状细胞的存在(可能是均匀的四边形细胞)和人多瘤病毒感染引起的染色体多倍体[35-37]。
本研究存在一些局限。首先,研究基于有症状的入院就诊人群,样本代表性有限,因此结果的普遍性需谨慎解读。其次,本研究仅比较了同一时间段内3种检测方法的结果,缺乏长期随访数据,限制了对检测方法长期效能的评估。此外,样本量相对较小,可能不足以全面评估统计学效力。未来研究应扩大样本量,特别是增加不同人群中的尿路上皮癌患者和正常对照群体,以便进行更全面和精确的分析。
综上所述,本研究比较了FISH、尿脱落细胞学以及二者联合检测在尿路上皮癌中的诊断效能。结果显示,在尿路上皮癌,特别是浸润性和高级别尿路上皮癌的诊断中,FISH的检测效能优于脱落细胞学;联合检测的准确率、灵敏度与FISH相当,但特异性较低。在非浸润性或低级别尿路上皮癌中,3种检测方法诊断效能均较低。FISH在尿路上皮癌的诊断中表现出较高的诊断价值,尤其适用于浸润性或高级别尿路上皮癌的检测。
利益冲突声明:所有作者均声明无利益冲突。
作者贡献声明:
王志婷:确定、设计选题,收集并分析数据,撰写文章;任敏,薛田,王皓晨,常恒:进行实验及判读;柏乾明,周晓燕:提供研究思路,分析研究方案可行性;朱晓丽:提供研究思路,分析研究方案可行性,参与文章的修订及最终版文章的审核。
[参考文献]
[1] BABJUK M, BURGER M, CAPOUN O, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ)[J]. Eur Urol, 2022, 81(1): 75-94.
[2] SUNG H, FERLAY J, SIEGEL R L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249.
[3] ROUPRÊT M, BABJUK M, BURGER M, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 update[J]. Eur Urol, 2021, 79(1): 62-79.
[4] 刘 坤. 超声对膀胱肿瘤诊断价值的评估[D]. 大连: 大连医科大学, 2018.
LIU K. Evaluation of ultrasonography in the diagnosis of bladder tumors [D]. Dalian Medical University, 2018.
[5] KONG C F, ZHANG S H, LEI Q F, et al. State-of-the-art advances of nanomedicine for diagnosis and treatment of bladder cancer[J]. Biosensors, 2022, 12(10): 796.
[6] LIU C L, TSAI H W, PENG S L, et al. CDCP1 (CUB domain containing protein 1) is a potential urine-based biomarker in the diagnosis of low-grade urothelial carcinoma[J]. PLoS One, 2023, 18(3): e0281873.
[7] YANG T, LI Y, LI J, et al. Diagnostic value comparison of urothelium carcinoma among urine exfoliated cells fluorescent in situ hybridization (FISH) examination, computerized tomography (CT) scan, and urine cytologic examination[J]. Med Sci Monit, 2018, 24: 5788-5792.
[8] PYCHA S, TRENTI E, MIAN C, et al. Diagnostic value of Xpert® BC Detection, Bladder Epicheck®, Urovysion® FISH and cytology in the detection of upper urinary tract urothelial carcinoma[J]. World J Urol, 2023, 41(5): 1323-1328.
[9] ZHANG J J, ZHENG S, GAO Y N, et al. A partial allelotyping of urothelial carcinoma of bladder in the Chinese[J]. Carcinogenesis, 2004, 25(3): 343-347.
[10] SOKOLOVA I A, HALLING K C, JENKINS R B, et al. The development of a multitarget, multicolor fluorescence in situ hybridization assay for the detection of urothelial carcinoma in urine[J]. J Mol Diagn, 2000, 2(3): 116-123.
[11] TODENHÖFER T, HENNENLOTTER J, ESSER M, et al. Stepwise application of urine markers to detect tumor recurrence in patients undergoing surveillance for non-muscle-invasive bladder cancer[J]. Dis Markers, 2014, 2014: 973406.
[12] GOMELLA L G, MANN M J, CLEARY R C, et al. Fluorescence in situ hybridization (FISH) in the diagnosis of bladder and upper tract urothelial carcinoma: the largest single-institution experience to date[J]. Can J Urol, 2017, 24(1): 8620-8626.
[13] LAVERY H J, ZAHARIEVA B, MCFADDIN A, et al. A prospective comparison of UroVysion FISH and urine cytology in bladder cancer detection[J]. BMC Cancer, 2017, 17(1): 247.
[14] AALAMI A H, AALAMI F. Diagnostic performance of fluorescence in situ hybridization (FISH) in upper tract urothelial carcinoma (UTUC): a systematic review and metaanalysis[J]. Int J Clin Oncol, 2022, 27(10): 1605-1615.
[15] JIN H Y, LIN T H, HAO J Q, et al. A comprehensive comparison of fluorescence in situ hybridization and cytology for the detection of upper urinary tract urothelial carcinoma: a systematic review and meta-analysis[J]. Medicine, 2018, 97(52): e13859.
[16] SCIARRA A, LASCIO G D, DEL GIUDICE F, et al. Comparison of the clinical usefulness of different urinary tests for the initial detection of bladder cancer: a systematic review[J]. Curr Urol, 2021, 15(1): 22-32.
[17] SASSA N, IWATA H, KATO M, et al. Diagnostic utility of UroVysion combined with conventional urinary cytology for urothelial carcinoma of the upper urinary tract[J]. Am J Clin Pathol, 2019, 151(5): 469-478.
[18] NAGAI T, OKAMURA T, YANASE T, et al. Examination of diagnostic accuracy of UroVysion fluorescence in situ hybridization for bladder cancer in a single community of Japanese hospital patients[J]. Asian Pac J Cancer Prev, 2019, 20(4): 1271-1273.
[19] TODENHÖFER T, HENNENLOTTER J, ESSER M, et al. Combined application of cytology and molecular urine markers to improve the detection of urothelial carcinoma[J]. Cancer Cytopathol, 2013, 121(5): 252-260.
[20] KNOWLES M A, HURST C D. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity[J]. Nat Rev Cancer, 2015, 15(1): 25-41.
[21] SHANG D H, LIU Y T, XU X H, et al. Diagnostic value comparison of CellDetect, fluorescent in situ hybridization (FISH), and cytology in urothelial carcinoma[J]. Cancer Cell
Int, 2021, 21(1): 465.
[22] COLLÀ RUVOLO C, WÜRNSCHIMMEL C, WENZEL M, et al. Comparison between 1973 and 2004/2016 World Health Organization grading in upper tract urothelial carcinoma treated with radical nephroureterectomy[J]. Int J Clin Oncol, 2021, 26(9): 1707-1713.
[23] STEWART B, WILD C. World cancer report 2014[M]. Lyon: IARC Press, 2014: 738-750.
[24] HALLING K C, KING W, SOKOLOVA I A, et al. A comparison of cytology and fluorescence in situ hybridization for the detection of urothelial carcinoma[J]. J Urol, 2000, 164(5): 1768-1775.
[25] MOONEN P M, MERKX G F, PEELEN P, et al. UroVysion compared with cytology and quantitative cytology in the surveillance of non-muscle-invasive bladder cancer[J]. Eur Urol, 2007, 51(5): 1275-1280; discussion1280.
[26] NAGAI T, NAIKI T, ETANI T, et al. UroVysion fluorescence in situ hybridization in urothelial carcinoma: a narrative review and future perspectives[J]. Transl Androl Urol, 2021, 10(4): 1908-1917.
[27] COMPÉRAT E, AMIN M B, BERNEY D M, et al. What’s new in WHO fifth edition - urinary tract[J]. Histopathology, 2022, 81(4): 439-446.
[28] SYDÉN F, BAARD J, BULTITUDE M, et al. Consultation on UTUC Ⅱ Stockholm 2022: diagnostics, prognostication, and follow-up-where are we today? [J]. World J Urol, 2023, 41(12): 3395-3403.
[29] SAROSDY M F, SCHELLHAMMER P, BOKINSKY G, et al. Clinical evaluation of a multi-target fluorescent in situ hybridization assay for detection of bladder cancer[J]. J Urol, 2002, 168(5): 1950-1954.
[30] HALLING K C, KING W, SOKOLOVA I A, et al. A comparison of BTA stat, hemoglobin dipstick, telomerase and Vysis UroVysion assays for the detection of urothelial carcinoma in urine[J]. J Urol, 2002, 167(5): 2001-2006.
[31] SAVIC S, ZLOBEC I, THALMANN G N, et al. The prognostic value of cytology and fluorescence in situ hybridization in the follow-up of nonmuscle-invasive bladder cancer after intravesical Bacillus Calmette-Guérin therapy[J]. Int J Cancer, 2009, 124(12): 2899-2904.
[32] 鲁颂献. 荧光原位杂交(FISH)技术在诊断尿路上皮癌中的研究 [D]. 吉林大学, 2013.
LU S X. Study on fluorescence in situ hybridization (FISH) technique in diagnosis of urothelial carcinoma [D]. Jilin University, 2013.
[33] 熊 钻, 梅玉峰, 王春阳, 等. 荧光原位杂交技术联合尿脱落细胞学、膀胱肿瘤抗原对尿路上皮细胞肿瘤的诊断效能分析[J]. 国际检验医学杂志, 2019, 40(10): 1201-1204.
XIONG Z, MEI Y F, WANG C Y, et al. Performance analysis of fluorescence in situ hybridization combined with urinary exfoliative cytology and bladder tumor antigen in the diagnosis of urothelial cell carcinoma[J]. Int J Lab Med, 2019, 40(10): 1201-1204.
[34] KE C J, HU Z Q, YANG C G. UroVysionTM fluorescence in situ hybridization in urological cancers: a narrative review and future perspectives[J]. Cancers, 2022, 14(21): 5423.
[35] MOATAMED N A, APPLE S K, BENNETT C J, et al. Exclusion of the uniform tetraploid cells significantly improves specificity of the urine FISH assay[J]. Diagn Cytopathol, 2013, 41(3): 218-225.
[36] DALQUEN P, KLEIBER B, GRILLI B, et al. DNA image cytometry and fluorescence in situ hybridization for noninvasive detection of urothelial tumors in voided urine[J]. Cancer, 2002, 96(6): 374-379.
[37] TAPIA C, GLATZ K, OBERMANN E C, et al. Evaluation of chromosomal aberrations in patients with benign conditions and reactive changes in urinary cytology[J]. Cancer Cytopathol, 2011, 119(6): 404-410.


